- Analysis is exploratory and has not been adjusted for multiple comparisons; no conclusions can be drawn
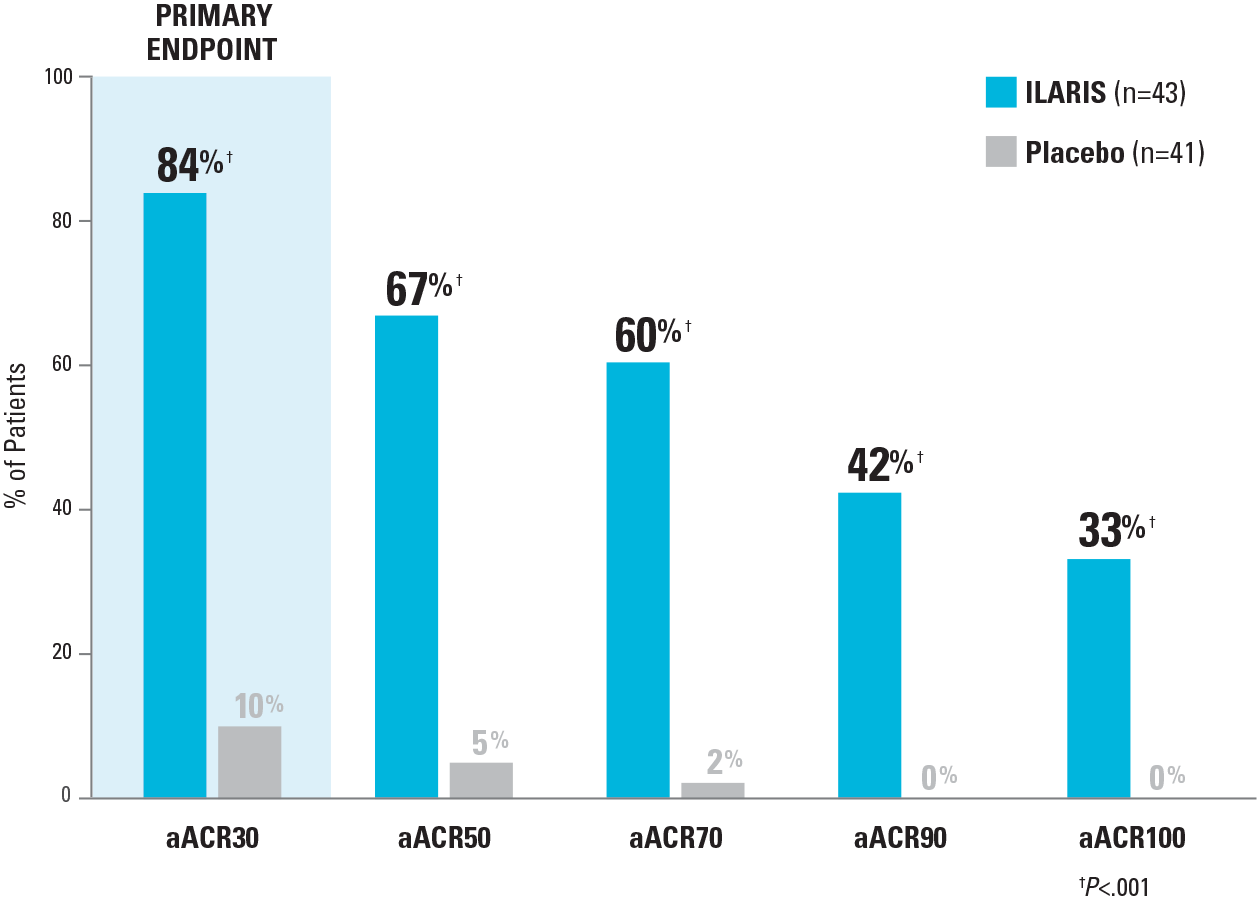
Significant improvements in aACR responses were seen with ILARIS at Day 151-3
aACR Responses* After the First Dose of ILARIS vs Placebo at Day 151-3
(n=43)
(n=41)
At Day 29, 81% (n/N=35/43) of patients receiving ILARIS compared with 10% (n/N=4/41) of patients receiving placebo achieved aACR30.3
HOC
ILARIS decreased steroid use and significantly reduced risk of flare1,3§

Of the 92 patients who attempted to taper their corticosteroids:
62% SUCCESSFULLY TAPERED|| THEIR STEROID DOSE (n/N=57/92)
ALMOST HALF (46%) WERE STEROID FREE (n/N=42/92)

Significant reductions in the risk of flare
64% REDUCTION IN RELATIVE FLARE RISK
74% PROBABILITY OF REMAINING FLARE§ FREE VS 25% WITH PLACEBO
- The study was ended after 37 flare events occurred. Median duration with ILARIS was 221.5 days vs 163.5 days with placebo. Hazard ratio was 0.36 (95% CI, 0.17-0.75)1,3,4
- The primary endpoint was corticosteroid tapering in at least 25% of patients being treated with corticosteroids (45% [57/128] were able to taper their doses of corticosteroids by the end of the steroid-tapering period in Study 2 [Part 1])1,3,4
- The primary endpoint was time to flare event with ILARIS vs placebo. This study continued until 37 flares had occurred1,3,4
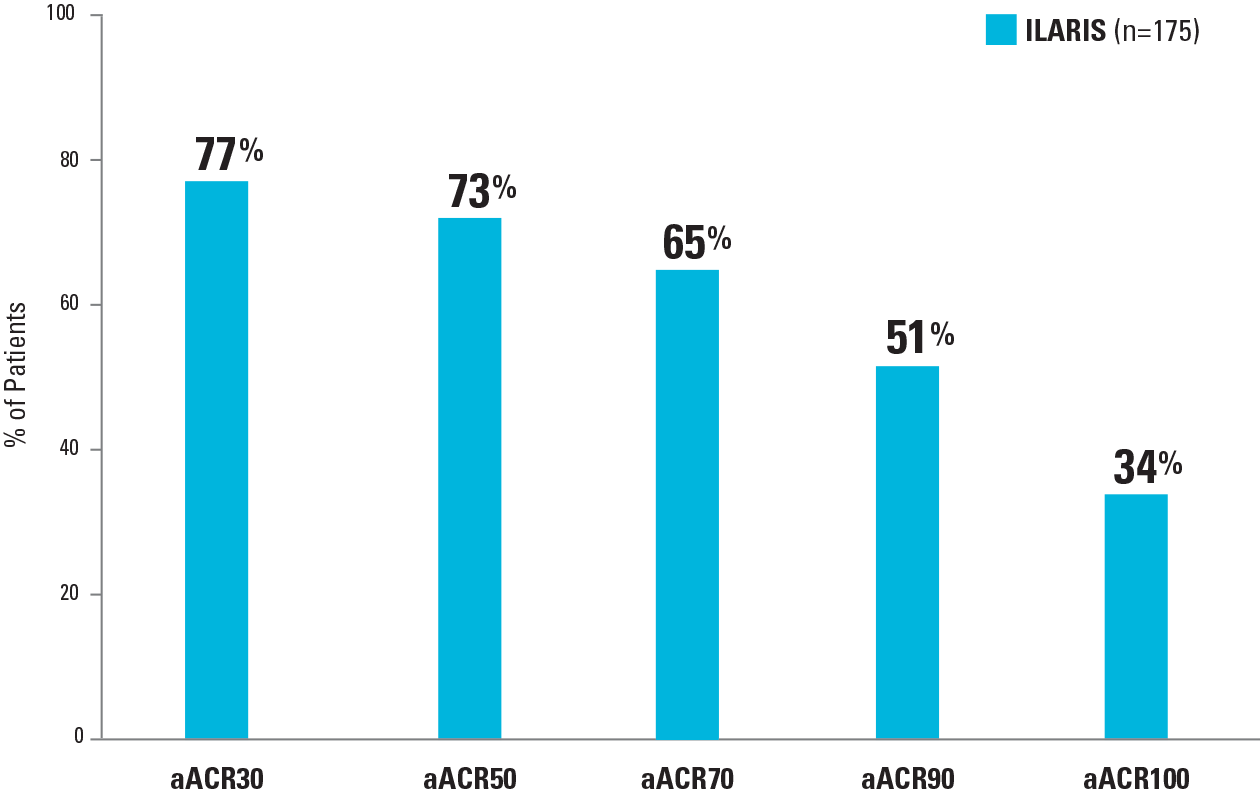
Many patients treated with ILARIS achieved aACR responses at the end of the study (≤8 months) and were fever free at Day 31,3,4
aACR Responses at the End of Study 2 (Part 1)
(N=175)
- End of Study 2 (Part 1) results shown are based on patients’ last available assessments
After the initial dose, nearly all patients in the ILARIS group were fever free at Day 3 of each pivotal trial2,4
Only 3 Days After Their First Dose of ILARIS:
- The primary endpoint was corticosteroid tapering in at least 25% of patients being treated with corticosteroids (45% [57/128] were able to taper their doses of corticosteroids by the end of the steroid-tapering period in Study 2 [Part 1])1,3,4
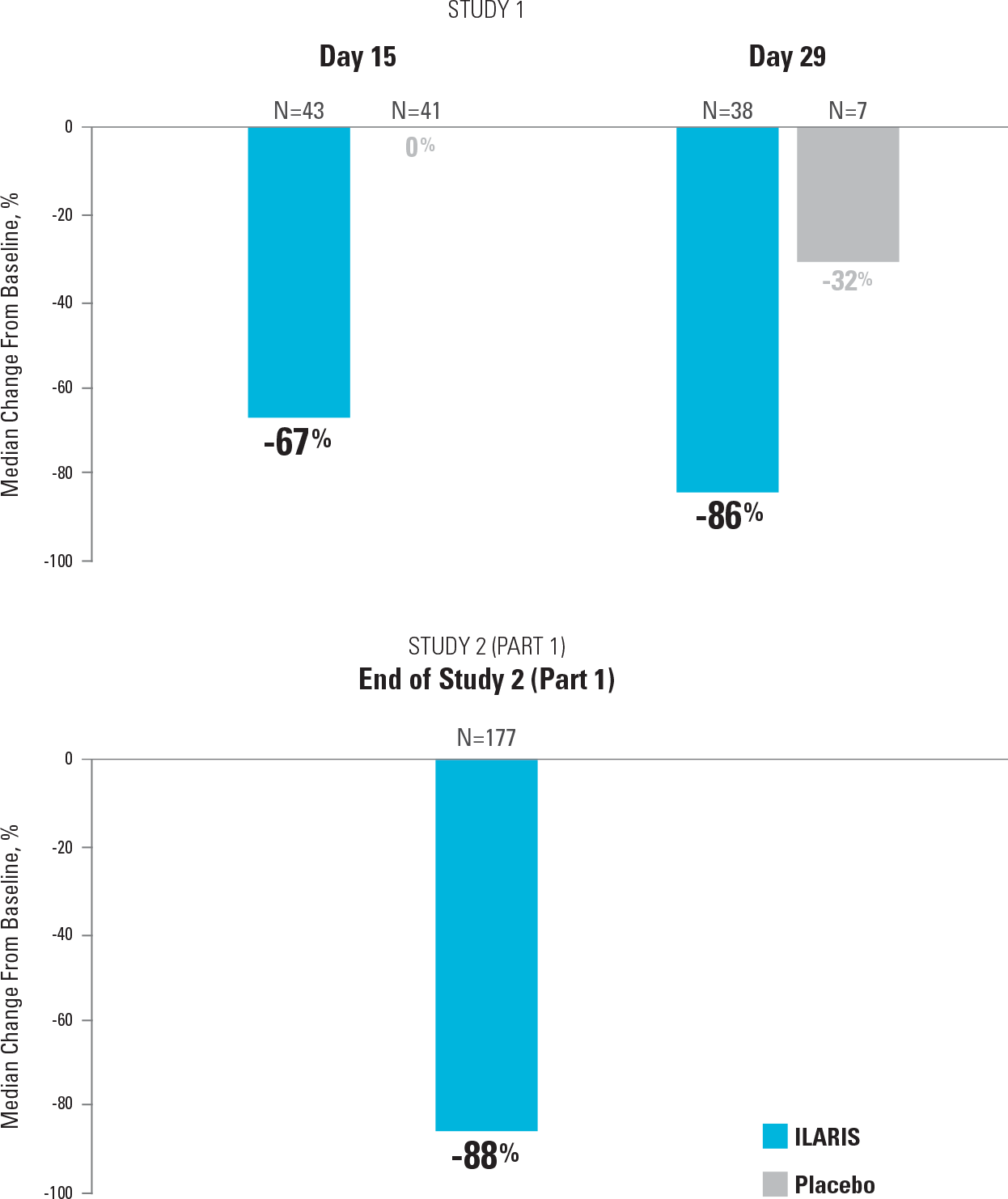
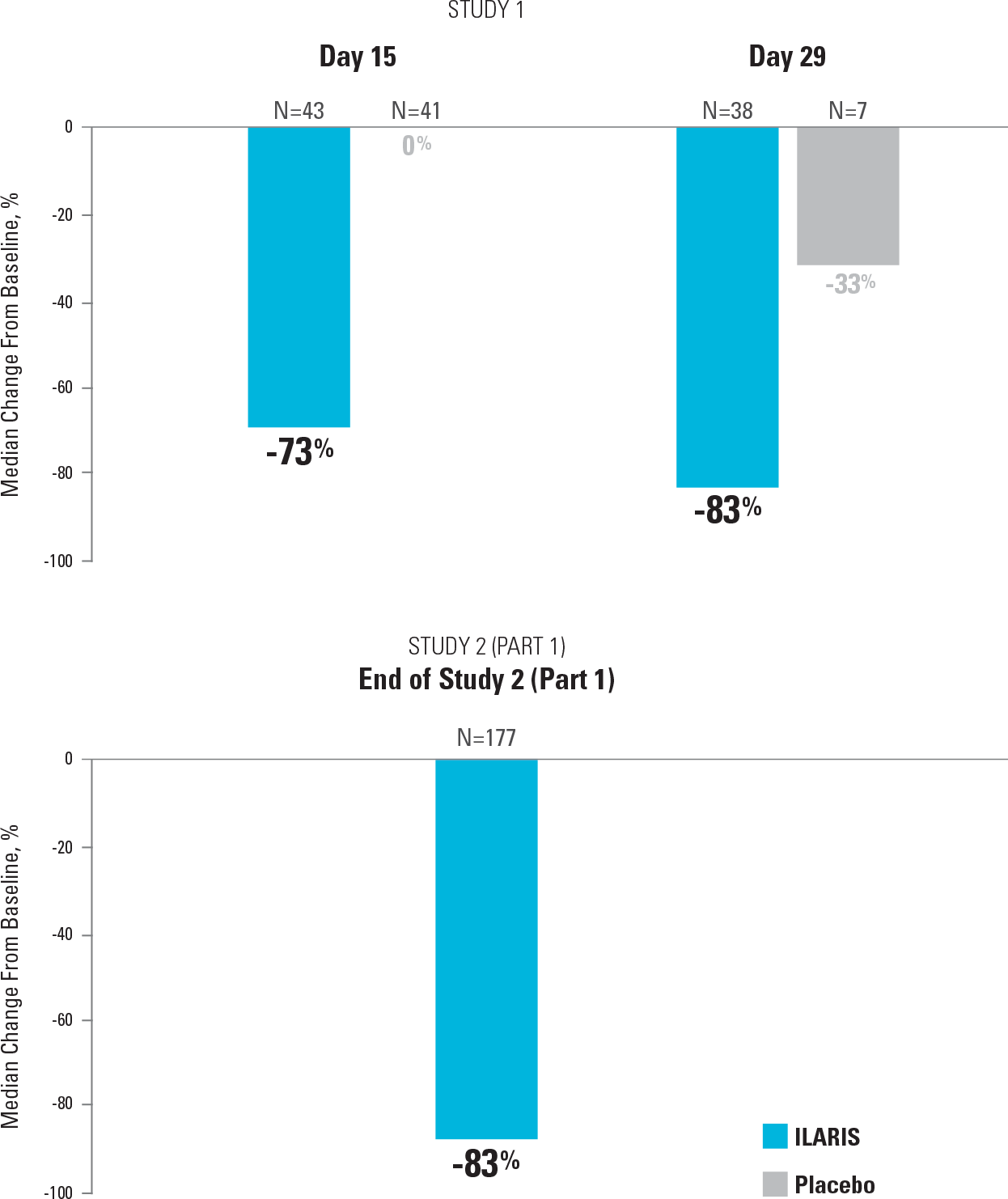
Once-monthly ILARIS provided systemic and arthritic improvements consistent with overall aACR response2,4
Median Reduction in Number of Active Arthritic Joints in Study 1 and Study 2 (Part 1)
(N=43)
(N=41)
(N=38)
(N=7)
(N=177)
Median Reduction in Number of Joints With Limited Range of Motion in Study 1 and Study 2 (Part 1)
(N=43)
(N=41)
(N=38)
(N=7)
(N=177)
Patients treated with ILARIS achieved a median reduction in CRP levels of approximately 90% in both pivotal trials.
- In Study 1, patients receiving ILARIS (n=43) achieved a 91% median reduction from baseline in CRP level at Day 15 vs a 5% median increase in patients receiving placebo (n=25)2
- In Study 2 (Part 1), patients receiving ILARIS (n=177) achieved an 87% median reduction from baseline in CRP level at the end of Study 2 (Part 1)4
The analysis of the aACR components have not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
The only FDA-approved treatment for AOSD¶
Efficacy of ILARIS was also assessed in a randomized, double-blind, placebo-controlled study that enrolled 36 patients (aged 22 to 70 years) diagnosed with AOSD
- The efficacy data were generally consistent with the results of a pooled efficacy analysis of patients with SJIA1
