Efficacy
Not an actual patient. Individual results will vary.
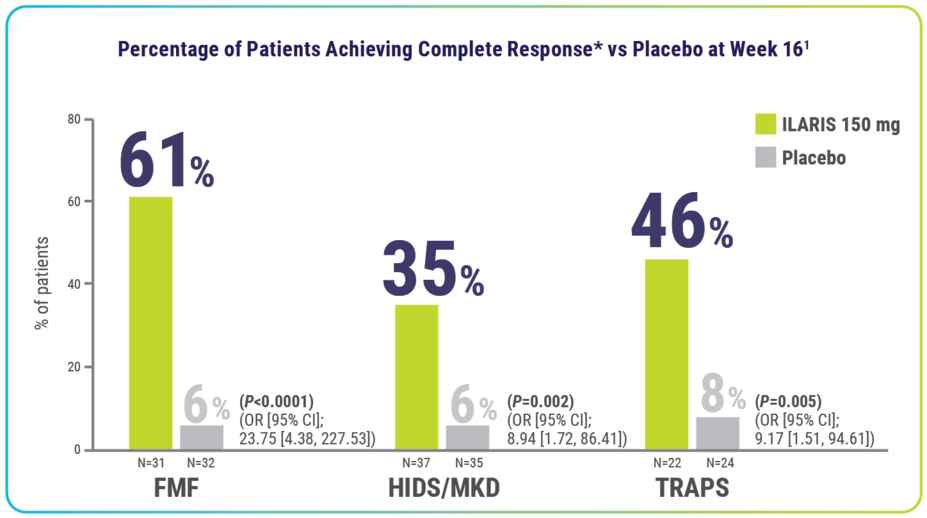
FMF, HIDS/MKD, and TRAPS Efficacy
Rapid resolution of index flare at Day 15—with no new flares through Week 16—was achieved by significantly more patients receiving ILARIS1
*Complete response defined as resolution of index flare (PGA <2 and CRP ≤10 mg/L or a ≥70% reduction from baseline) at Day 15 and no new flare (PGA ≥2 and CRP ≥30 mg/L) throughout the 16-week treatment period.1
At Day 15, the majority of patients with FMF (81%, n/N=25/31), HIDS/MKD (65%, n/N=24/37), and TRAPS (64%, n/N=14/22) who received ILARIS achieved resolution of index disease flare vs placebo: FMF (31%, n/N=10/32), HIDS/MKD (37%, n/N=13/35), and TRAPS (21%, n/N=5/24).1
Signs of disease activity
After the initial dose at Day 15, ILARIS improved disease activity as measured by PGA, as well as by CRP levels1
PGA scores at baseline2
| FMF | HIDS/MKD | TRAPS |
| 10% in the ILARIS group had mild disease vs 19% in the placebo group2 | 27% in the ILARIS group had mild disease vs 20% in the placebo group2 | 41% in the ILARIS group had mild disease vs 46% in the placebo group2 |
| In the ILARIS group, 55% had moderate disease and 36% had severe disease compared with 59% and 22%, respectively, in the placebo group | In the ILARIS group, 60% had moderate disease and 14% had severe disease compared with 60% and 20%, respectively, in the placebo group | In the ILARIS group, 50% had moderate disease and 9% had severe disease compared with 46% and 8%, respectively, in the placebo group |
At Day 15, CRP ≤10 mg/L achieved by1:
| FMF | HIDS/MKD | TRAPS |
90% of patients receiving ILARIS (n/N=28/31) vs 28% receiving placebo (n/N=9/32)
| 68% of patients receiving ILARIS (n/N=25/37) vs 26% receiving placebo (n/N=9/35)
| 59% of patients receiving ILARIS (n/N=13/22) vs 33% receiving placebo (n/N=8/24)
|
CAPS Efficacy
The majority of patients in Part 1 achieved complete clinical response at Weeks 1 and 8 after the first dose of ILARIS1,4
| WEEK 1 | WEEK 8 |
| 71% | 97% |
| (n/N=25/35) | (n/N=34/35) |
Complete clinical response was defined as meeting all of the following criteria4:
- Physician’s assessment of disease activity ≤ minimal (rated on a 5-point scale consisting of absent, minimal, mild, moderate, and severe)
- Assessment of skin disease ≤ minimal (rated on a 5-point scale consisting of absent, minimal, mild, moderate, and severe)
- Normal serum values of CRP and SAA (<10 mg/L)
Assessment of disease activity included a composite of the following symptoms: urticarial skin rash, headache/migraine, fatigue/malaise, conjunctivitis, arthralgia, myalgia, and other symptoms related or unrelated to CAPS.
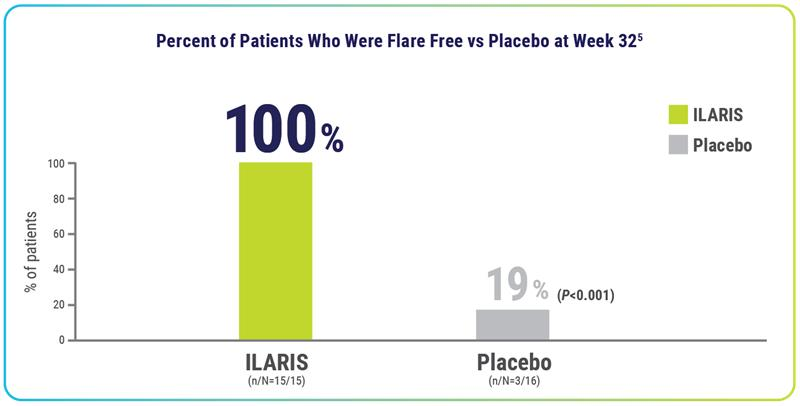
Flare reduction
After 3 doses† of ILARIS in Part 2, 100% of patients remained flare free through 24 weeks1,4‡
Disease relapse
None of the patients treated with ILARIS had a disease relapse§ past 24 weeks4
- For patients taking placebo, median time to flare was 100 days
- During the open-label treatment period (Part 3), 97% (n/N=30/31) of patients were without disease relapse5||
Physician’s Global Assessment of Disease Activity and Assessment of Skin Disease1,4
| Part 2 | Part 3 | ||
| ILARIS | Placebo | ILARIS | |
| No rash | 93% (n/N=14/15) | 31% (n/N=5/16) | 94% (n/N=29/31) |
| Minimal rash | 7% (n/N=1/15) | 19% (n/N=3/16) | 6% (n/N=2/31) |
| No or minimal disease activity | 100% (n/N=15/15) | 25% (n/N=4/16) | 97% (n/N=30/31) |
Analysis has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
*Complete response defined as resolution of index flare (PGA <2 and CRP ≤10 mg/L or a ≥70% reduction from baseline) at Day 15 and no new flare (PGA ≥2 and CRP ≥30 mg/L) throughout the 16-week treatment period.1
†For patients with CAPS, ILARIS is dosed once every 8 weeks.1
‡ Ten patients in the placebo group met the criteria for clinical relapse, and 3 patients discontinued Part 2 due to unsatisfactory therapeutic effect.4
§Disease relapse: Defined as CRP and/or SAA value >30 mg/L and either a score of mild or worse for physician’s assessment of disease activity, or a score of minimal or worse for physician’s assessment of disease activity and assessment of skin disease.1
||Includes all 15 patients randomized to ILARIS in Part 2 and 15 of 16 patients randomized to placebo in Part 2. Disease relapse was defined as CRP and/or SAA value >30 mg/L and either a score of mild or worse for physician’s assessment of disease activity, or a score of minimal or worse for physician’s assessment of disease activity and assessment of skin disease.1,4
References: 1. Ilaris. Prescribing information. Novartis Pharmaceuticals Corp. 2. Data on file. CACZ885N2301 FMF, HIDS/MKD, and TRAPS Clinical Study Report. Novartis Pharmaceuticals Corp; 2016. 3. De Benedetti F, Gattorno M, Anton J, et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med. 2018;378(20):1908-1919. 4. Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, et al; Canakinumab in CAPS Study Group. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360(23):2416-2425. doi:10.1056/NEJMoa0810787 5. Data on file. CACZ885D2304 CAPS Clinical Study Report. Novartis Pharmaceuticals Corp; 2009.