![[Left] Woman contemplating the luck her four-leaf clover necklace could bring her. [Right] Four-leaf clover necklace left behind.](https://usim.beprod.ilarishcp.com/sites/ilarishcp_com/files/styles/hero_full_width_width_2560/public/2024-09/stills-mechanism-of-action-hero-image-d_1.jpg?itok=0yMgLxKb)
Efficacy
Not an actual patient. Individual results will vary.
Rapid relief isn’t luck — it’s ILARIS
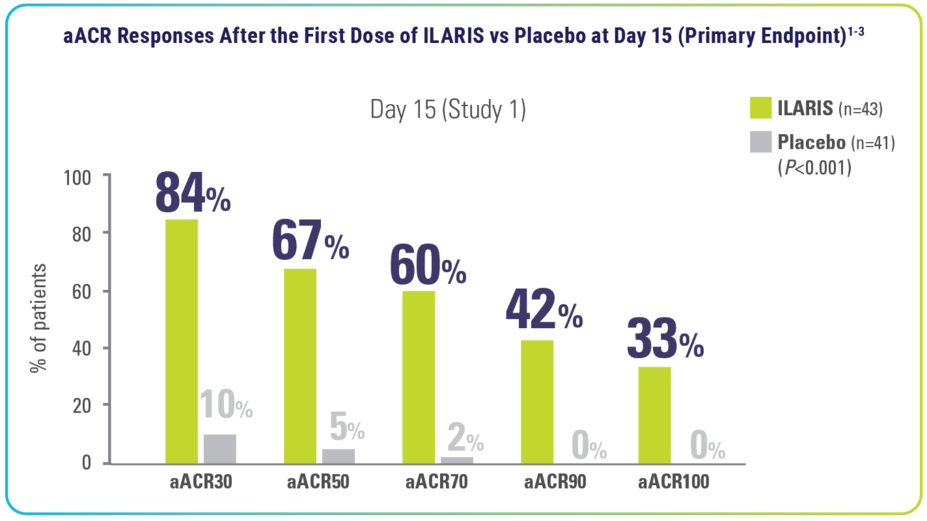
Rapid aACR responses* across arthritic and systemic manifestations1-3
Long-term aACR responses* across arthritic and systemic manifestations up to Month 83,4†
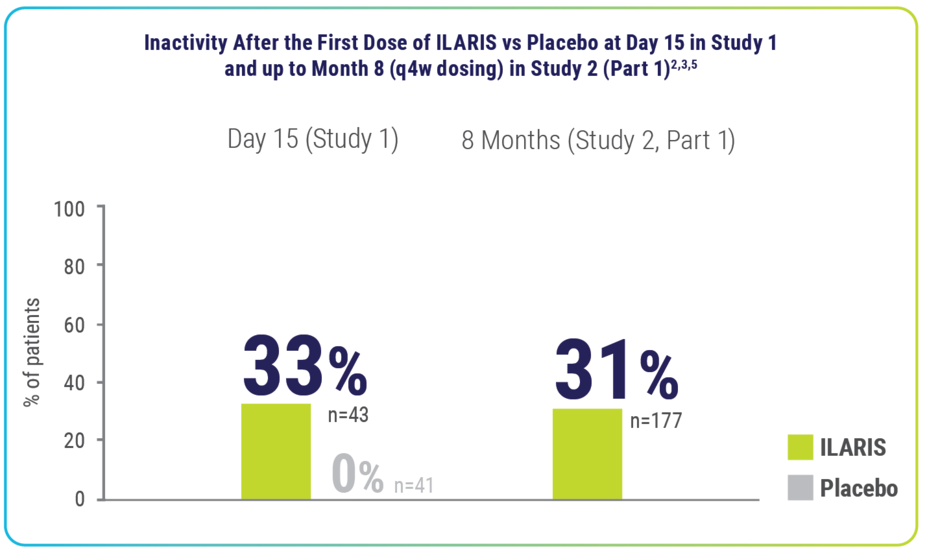
In an exploratory post hoc analysis, remission‡ was observed in 33% of patients taking ILARIS2,3,5
Rapid disease inactivity‡ was observed at Day 15 and long-term (up to 8 months) disease inactivity was seen on ILARIS2,3,5
- For 1 in 3 patients, disease inactivity‡ was observed after 15 days of starting treatment with ILARIS (vs none with placebo)2,3,5
Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions can be drawn.
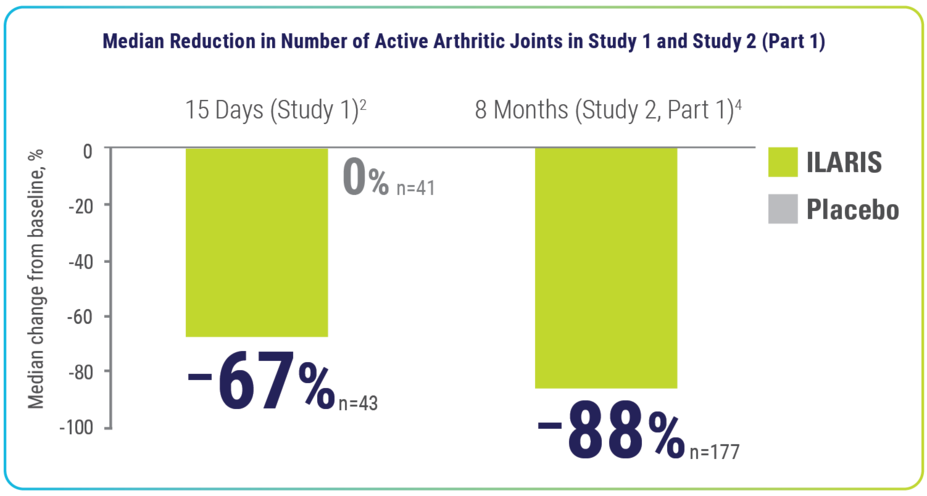
ILARIS delivered rapid relief from arthritic manifestations that improved over time2-4
Within 15 days and up to 8 months, ILARIS reduced the number of active arthritic joints2,4
Median number of active arthritic joints at baseline3:
- Trial 1: Placebo, 7.0; ILARIS, 10.0
- Trial 2: ILARIS, 10.0
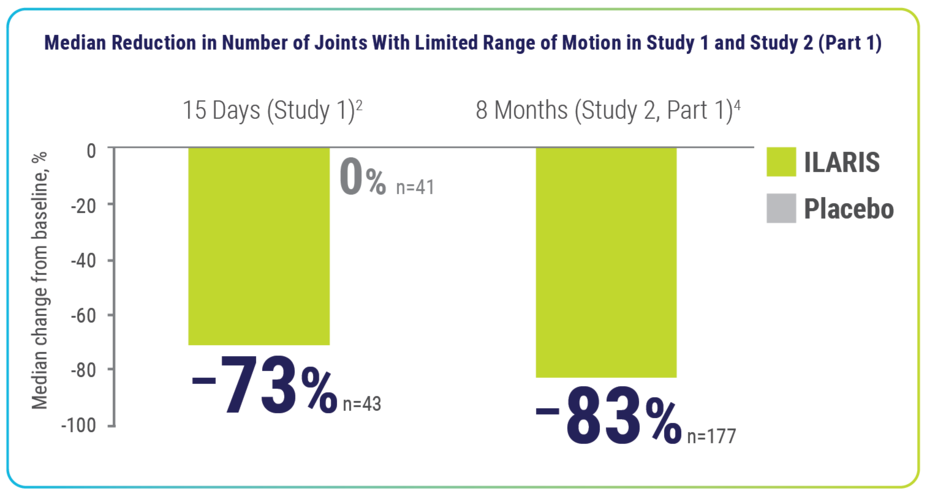
Within 15 days and up to 8 months, ILARIS reduced the number of joints with limited range of motion2,4
Median number of joints with limited range at baseline3:
- Trial 1: Placebo, 6.0; ILARIS, 8.0
- Trial 2: ILARIS, 9.0
Results for the systemic and arthritic components of the pediatric aACR core set were consistent with the overall aACR response results.1
The analysis of the aACR components have not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
ILARIS delivered rapid relief from systemic manifestations
After the initial dose, nearly all patients taking ILARIS were fever free at Day 32,4
| STUDY 12 | STUDY 2 (Part 1)4 |
100% of patients were fever free (n=43/43) vs 87% with placebo (n=33/38) | 99% of patients were fever free (n=139/141) |
Patients treated with ILARIS achieved a median reduction in CRP levels of approximately 90%2,4
- In Study 1, patients receiving ILARIS (n=43) achieved a 91% median reduction from baseline in CRP level at Day 15 vs a 5% median increase in patients receiving placebo (n=25)2
- In Study 2 (Part 1), patients receiving ILARIS (n=177) achieved an 87% median reduction from baseline in CRP level at the end of Study 2 (Part 1)4
- Results for the systemic and arthritic components of the pediatric aACR core set were consistent with the overall aACR response results1
The analysis of the aACR components have not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
ILARIS decreased steroid use and significantly reduced the risk of flare1,3§
Of the 92 patients who attempted to taper their corticosteroids‖: 62% of patients successfully tapered¶ their steroid use (n/N=57/92) and 46% discontinued use entirely (n/N=42/92)1
ILARIS demonstrated a 64% relative reduction in flare risk compared with placebo for disease control1:
- The study was ended after 37 flare events occurred. Median duration with ILARIS was 221.5 days vs 163.5 days with placebo. Hazard ratio was 0.36 (95% CI, 0.17-0.75)1,3,4
ILARIS was studied in multiple trials of SJIA patients1-4
| Description | Population | Dosing | Primary Endpoint |
| Study 11-3 | |||
| Randomized, double-blind, placebo-controlled study | 84 patients with SJIA | Single subcutaneous dose of ILARIS (4 mg/kg) vs placebo over 29 days | aACR30 at Day 15 |
| Study 2 (Part 1) 1,3,4 | |||
Open-label steroid-tapering phase
| 177 patients | 4-mg/kg subcutaneous dose of ILARIS every 4 weeks for 12 to 32 weeks | Corticosteroid tapering in at least 25% of patients being treated with corticosteroids
|
| Study 2 (Part 2)1,3,4 | |||
| A double-blind withdrawal trial | Patients from Study 2 (Part 1), who achieved and sustained aACR30 or above in Part 1 and were not taking corticosteroids or who had undergone successful corticosteroid tapering | 4 mg/kg of ILARIS (n=50) or placebo (n=50) every 4 weeks | Time to flare event with ILARIS vs placebo |
The efficacy of ILARIS in adults with AOSD is based on the pharmacokinetic exposure and extrapolation of the established efficacy of ILARIS in SJIA patients. Efficacy of ILARIS was also assessed in a randomized, double-blind, placebo-controlled study that enrolled 36 patients (22 to 70 years old) diagnosed with AOSD. The efficacy data were generally consistent with the results of a pooled efficacy analysis of SJIA patients.1
*aACR response: Percentage improvement (at least 30%, 50%, 70%, 90%, or 100%) from baseline in at least 3 of the 6 pediatric ACR core outcome components along with the absence of fever (≤38 °C in the preceding 7 days) and worsening of >30% in no more than 1 of the remaining components. The disease activity components include CRP level, number of joints with active arthritis, number of joints with limited range of motion, physician’s global assessment of disease activity, parent’s or patient’s global assessment of patient’s overall well-being, and functional ability (CHAQ-DI).2,3
†End of Study 2 (Part 1) results shown are based on patients’ last available assessments.4
‡Remission (inactive disease): Absence of active arthritis, fever, rheumatoid rash, serositis, splenomegaly, hepatomegaly, or generalized lymphadenopathy attributable to SJIA; normal ESR or CRP; physician’s global assessment of disease activity indicating no disease activity. P values were not determined for comparison regarding inactive disease.2,3,5
§Flare: Worsening of ≥30% in at least 3 of the 6 core aACR response variables combined with improvement of ≥30% in no more than 1 of the 6 variables, or reappearance of fever not due to infections for at least 2 consecutive days.1
‖92 of the total 128 patients taking corticosteroids who entered the open-label portion of Study 2.1
¶Successful corticosteroid tapering: Oral prednisone (or equivalent) dose reduction from >0.8 to ≤0.5 mg/kg/day, or from ≥0.5 and ≤0.8 mg/kg/day by at least 0.3 mg/kg/day, or from any initial dose to ≤0.2 mg/kg/day, while maintaining a minimum aACR30 response.4
References: 1. Ilaris. Prescribing information. Novartis Pharmaceuticals Corp. 2. Data on file. CACZ885G2305 SJIA Study 1 Clinical Study Report. Novartis Pharmaceuticals Corp; 2011. 3. Ruperto N, Brunner HI, Quartier P, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2396-2406. doi:10.1056/NEJMoa1205099 4. Data on file. CACZ885G2301 SJIA Study 2 Clinical Study Report. Novartis Pharmaceuticals Corp; 2012. 5. Wallace CA, Ruperto N, Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31(11):2290-2294.